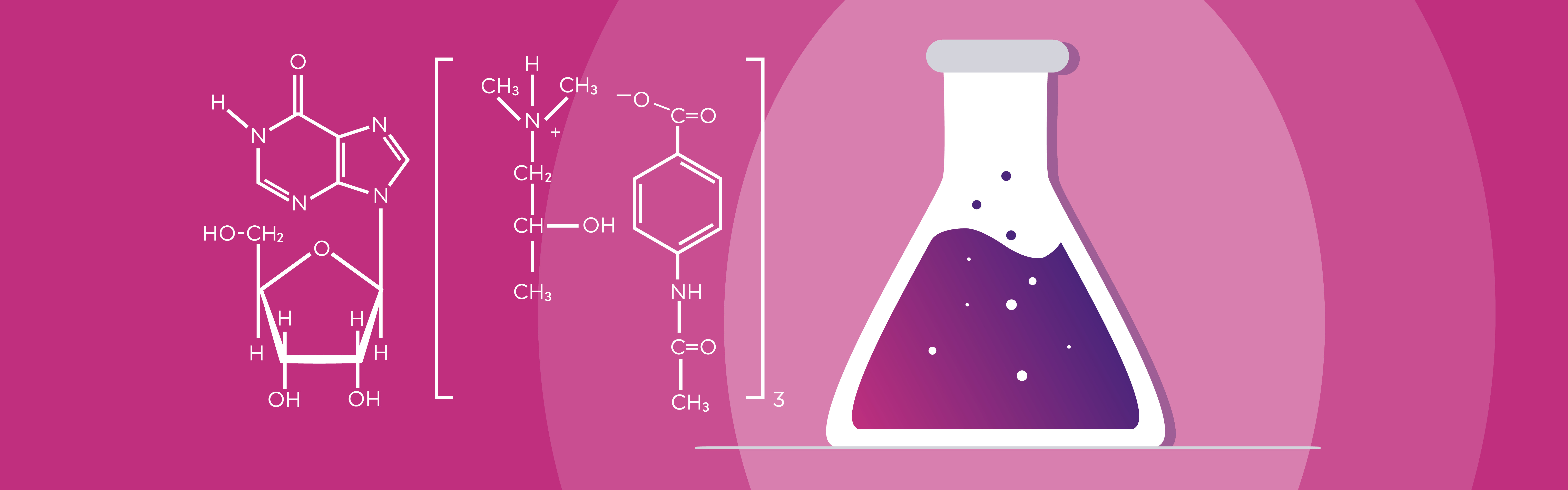
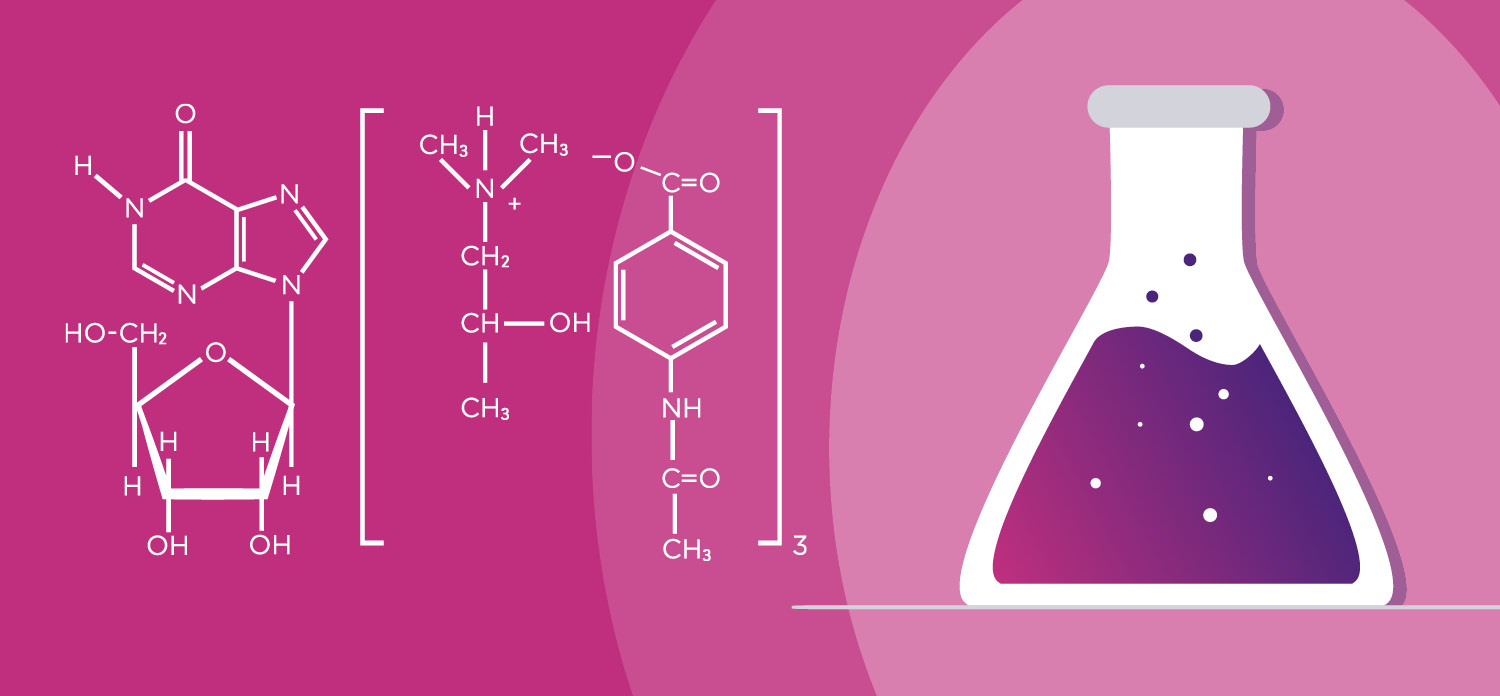
UNIQUE DUAL MODE OF ACTION
Viruxan® (inosine acedoben dimepranol) with unique proven double effect restores the immune system and stimulates the antiviral defence in the human body.¹ The active substance in Viruxan® Forte is the original IAD molecule with its well-established safety profile and more than 50 years of real-life experience. IAD is a synthetic purine derivative of inosine. Inosine is a natural purine that is present in foods such as meat and meat extracts.1,5 The first patent for the synthetic purine derivate, Inosine Acedoben Dimepranol, was granted to the US chemist Dr. Paul Gordon in 1972.


THE ANTIVIRAL EFFECT
- Once a virus has entered a human cell, Viruxan® inhibits the replication process of the virus' genetic material (RNA).
- IAD's antiviral activity is non-specific and is therefore effective against various viruses.²

THE IMMUNITY EFFECT
- Viruxan® heightens immune function and increases the number and function of NK cells.
- Just 90 minutes after the intake of Viruxan®, the number of NK cells increases as a percentage of total peripheral blood lymphocytes.
- After five days, the number of NK cells is doubled, and the immune system can work more effectively.³
CLINICALY PROVEN EFFICACY
Viruxan® Forte reduces the symptoms and duration of acute viral respiratory infections: In case of an acute viral respiratory infection, Viruxan® Forte can restore proper immune function, significantly reduce symptomatology, and shorten the duration of symptoms by two days in patients below 50 years of age (Figure 1).4
Figure 1: Faster time to resolution of all influenza-like symptoms in patients below 50 years of age in IAD treatment group. Adapted from Beran J, Šalapova E, Špajdel M; Isoprinosine Study (EWO ISO-2014/1) Team. Inosine acedoben dimepranol is safe and effective for the treatment of subjects with confirmed acute respiratory viral infections: analysis and subgroup analysis from a Phase 4, randomised, placebo-controlled, double-blind study. BMC Infect Dis. 2016 Nov 7;16(1):648. doi: 10.1186/s12879-016-1965-5. PMID: 27821093; PMCID: PMC510017
References:
1. Sliva J, Pantzartzi CN, Votava M. Inosine Pranobex: A Key Player in the Game Against a Wide Range of Viral Infections and Non-Infectious Diseases. Adv Ther. 2019 Aug;36(8):1878-1905. doi: 10.1007/s12325-019-00995-6. Epub 2019 Jun 5. PMID: 31168764; PMCID: PMC6822865; 2. CCDS Viruxan 2020; 3. Rumel Ahmed S, Newman AS, O’Daly J, Duffy S, Grafton G, Brady CA, John Curnow S, Barnes NM, Gordon J. Inosine Acedoben Dimepranol promotes an early and sustained increase in the natural killer cell component of circulating lymphocytes: A clinical trial supporting anti-viral indications. Int Immunopharmacol. 2017 Jan;42:108-114.doi: 10.1016/j.intimp.2016.11.023. Epub 2016 Nov 29. PMID: 27912146; 4. Beran J, Šalapová E, Špajdel M; Isoprinosine Study (EWO ISO-2014/1) Team. Inosine pranobex is safe and effective for the treatment of subjects with confirmed acute respiratory viral infections: analysis and subgroup analysis from a Phase 4, randomised, placebo-controlled, double-blind study. BMC Infect Dis. 2016 Nov 7;16(1):648. doi: 10.1186/s12879-016-1965-5. PMID: 27821093; PMCID: PMC5100179; 5. Bulgakova VA, Balabolkin II, Katosova LK, Sedova MS, Zubkova IV. Assessment of the efficacy of combined effect immunomodulator inosine pranobex in preventing respiratory infections in children with allergies. Pediatric pharmacology. 2010; 7(5): 30-37